Early Stage Development
Maximize Label Value | Optimize Resources | Manage Risk
You may not be ready to hire a chief commercial officer, but be sure you build into your development plans the critical commercial check-points so your product development team and executive panels are informed. With our Early Stage Development solution, the early phases are low-investment and high-value.
Are Your Early Stage Development Plans Complete?
With clinical and supply plans in hand, early stage development teams are focused and confident in their asset. Most lack early commercial plans, thereby overlooking the critical commercial components that are necessary to optimize a label for launch.
Minimize Risk
In today's environment, developing a product without being informed of the market (payer, provider, patient, and competitors) is risky. Commercial failures often root in poor or non-existent commercial input during the early phases of development. Without an early-stage commercial plan, development teams lack the critical market perspectives to adequately design clinical trials that will maximize their product's value, minimize the product's risk in the market, or get through regulatory with the label needed.
The ForeRunner Strategy advisors will work with you to create a customized early-stage commercial plan. With our customized plan, your team can make informed decisions as to when payers and providers are engaged and when to bring in marketing help.
Phase 3 Is Too Late
Today, commercial planning cannot be ignored in early phases of development. An integrated plan outlines the needed market perspectives in the design and development of market-relevant trial end-points that will satisfy payers, providers, patients, and regulators. Don't ignore what you don't know. Waiting until Phase 3 to integrate commercial plans with your clinical and manufacturing plans will inevitably leave opportunity on the table, miss the mark with stakeholders, and risk launch disasters.
Be prepared for the next round of funding
Whether your goal is to take your product to market or exit, be sure your product development plans have a viable commercial planning component. Investors, buyers, and partners are more likely to take your product seriously when you are prepared with viable commercial components in your development plans.
IPL™ Early Stage Commercial Solutions
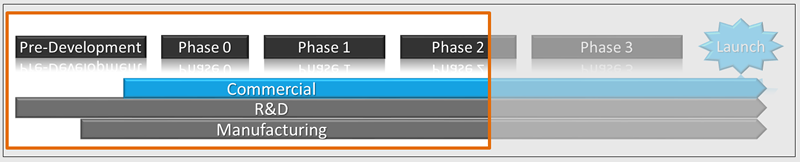
IPL™ early stage commercial solutions line up commercial activities along side those of clinical and supply, allowing development teams to understand the market, design clinical trials to build a label that is responsive to market needs and maximizes the asset's value, and proactively manage necessary commercial touch-points.
IPL™ plans inform your product development teams and executive panels when critical commercial input is necessary:
We'll work with your team to incorporate critical commercial components into your plans, develop customized dashboards to monitor and track progress, and make sure your plans are integrated with clinical and supply efforts.
Benefit from our experience and from those who have gone before you
Call or email us and we'll answer any questions you have about how IPL™ can work for you.